Different substances provide differing levels of electrical conductivity, depending on multiple factors, including the composition of the material. A semiconductor provides low levels of conductivity for electric current, with the precise amount of resistance determined by the specific type of substance. Semiconductors can be elements, compounds, or layered compounds, each of which offers a different level of conductivity.
Semiconductor Definition
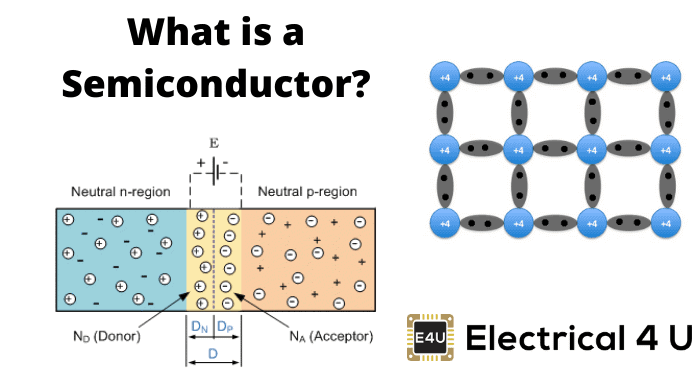
Electrical current is passed through substances at different rates, depending on the level of resistance to electricity found in the material. All materials fall into one of three categories when it comes to electrical conductivity.
- An insulator does not allow any electricity to pass through it at all.
- A superconductor offers little or no resistance, and electricity passes through it swiftly.
- A semiconductor allows electrical current to pass, but at reduced rates.
Semiconductors are, therefore, materials that allow a limited amount of electrical current to pass through them, but not at the same rates provided by superconductors.
Types of Semiconductors

Types of semiconductors include compounds, elements, and layered compounds. Each type of semiconductor is commonly used for different purposes, and created, when necessary, in specific ways to suit those purposes.
- Semiconductor Elements– Elements are substances that are made of only one type of atom, and cannot be broken down into other substances. Elements that are commonly used as semiconductors include: silicon, germanium, and tin. Silicon is frequently used in electronics, due to its semiconducting properties.
- Semiconductor Compounds– Compounds, as the name implies, are substances that are a combination of 2 or more elements. A compound semiconductor commonly used in electronics is GaAs, or Gallium Arsenide. Gallium is a very rare substance, and is combined with Arsenic to make Gallium Arsenide (GaAs). Gallium Arsenide is another semiconductor compound often used in electronics, and increasingly in solar cells.
- Layered Compound Semiconductors– Layered compounds are attached together with a covalent bonding process. A covalent bond occurs when the substances are aligned in a manner in which the layers share electrons. One example of layered compounds is the combination of GaSe (Gallium Selenide) and GaTe (Gallium Telluride).
Electrical Conductivity
The amount of electrical resistance provided by an object depends on more than just its composition. Temperature and size also change the amount of resistance offered by the object. For example, an electrical current sent through a long wire will encounter more resistance than the same current through a short wire. In addition, lower temperatures reduce the amount of resistance to electrical current.